There were three goals for this project.
- Develop a device for taking samples in various parts of the gastrointestinal tract
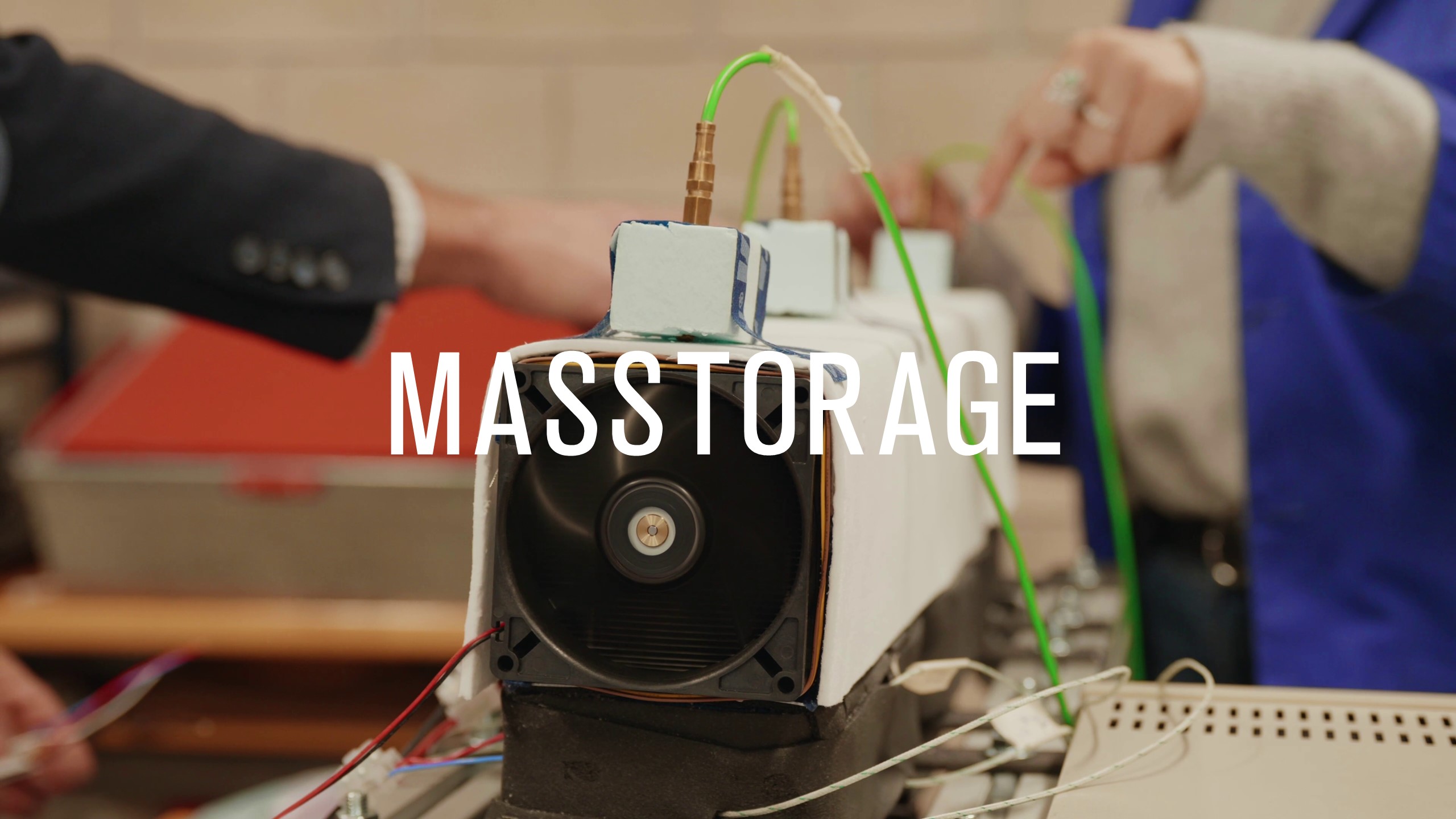
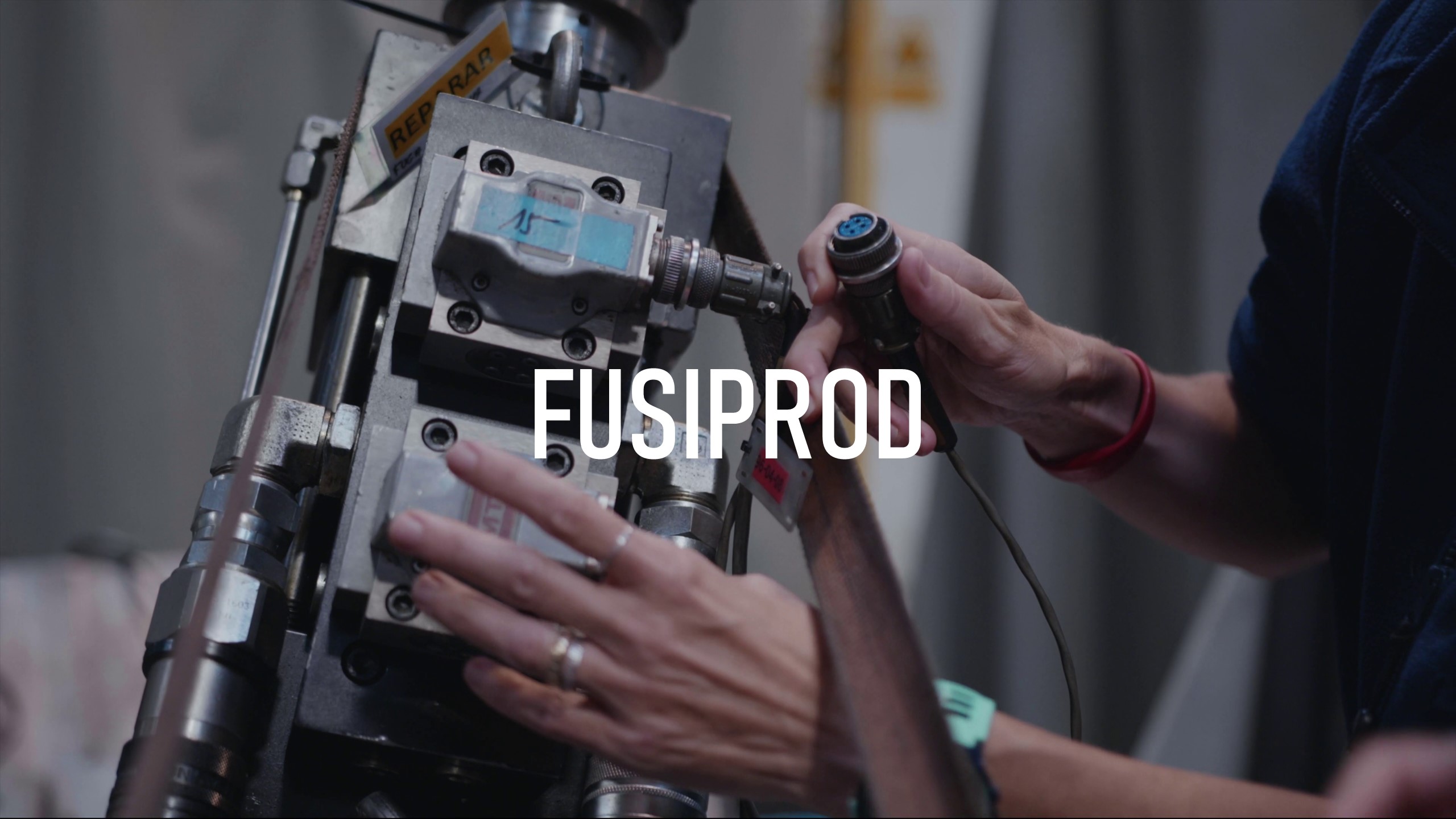
The goal of this task was to design a capsule for taking samples that met the functional requirements and regulations in force. Work was done sequentially on the parameters that made it possible to design, fabricate and test the sample
taking device.
In regard to the material, the following parameters were taken into consideration.
- it has to be biodegradable and gastro-resistant, to withstand the entire trajectory from the mouth to the anus, and it has to be resistant to the environment.
- It has to be biocompatible and not release toxic substances.
- It must have the properties necessary to be easily injectable.
- It must be compatible with 3D printed moulds: have a good injection temperature.
so it does not damage the mould, and a good fluidity index or low viscosity. - It must have properties suitable to withstand the mechanical shooting mechanism.
- It must be able to protect the sample taken and its components.
- It must be able to take a good finish and meet the tolerances well, so it can be assembled.
- It must be economically accessible.
For that reason, polypropylene, poly (methyl methacrylate), polycarbonate and ABS was used.
The designs were tested and tuned in two kinds of gastrointestinal tract models.
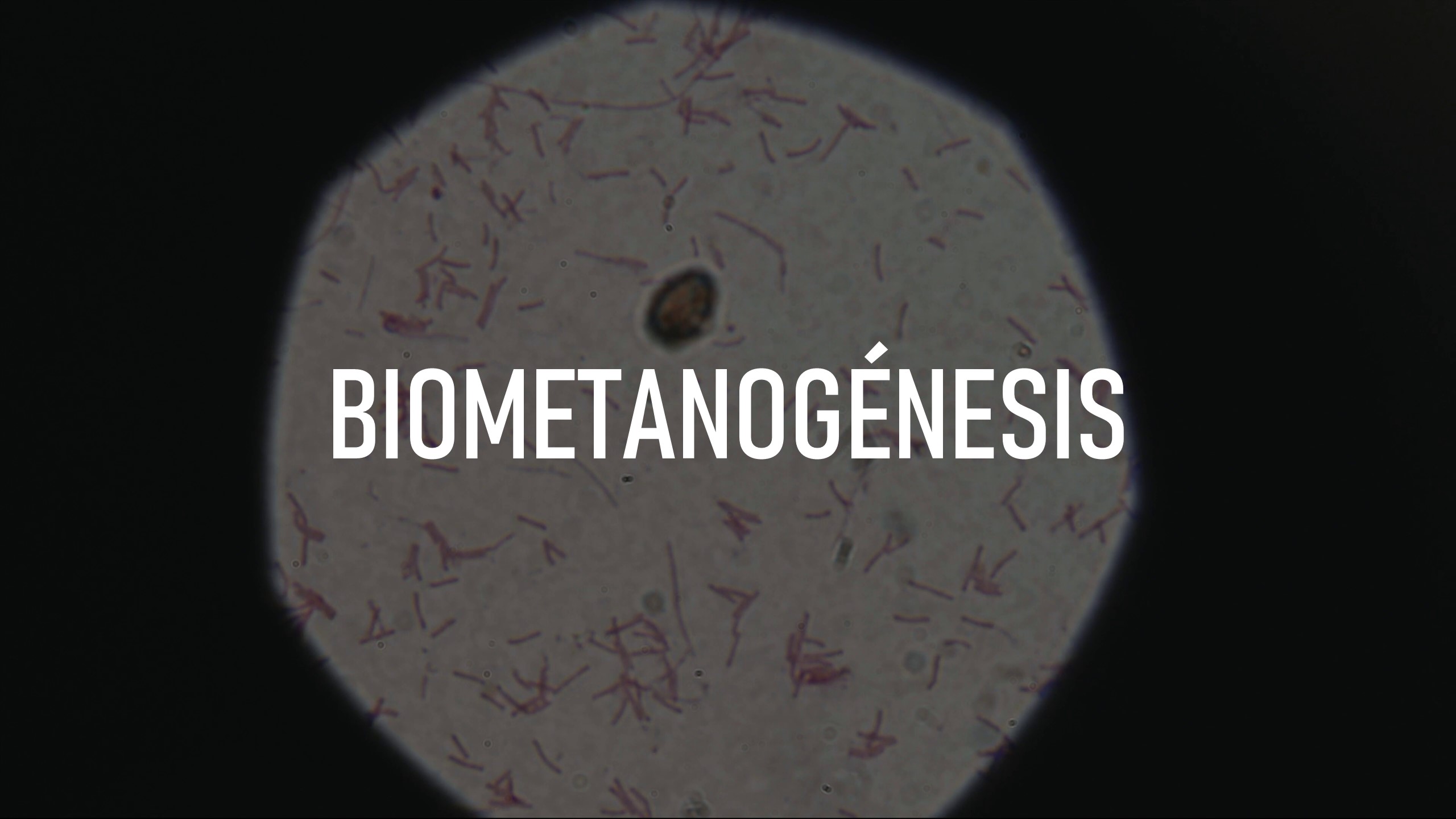
Model 1: In vitro bioreactor at the CAF (food science) department of the UNAV, which made it possible to use faeces or microbiome samples. And, with suitable measurements and temperature, pH and nutrient conditions, conditions could be simulated in different parts of the human gastrointestinal tract, including in vitro gastrointestinal digestion (acid and enzyme) and the action of intestinal microbiome.
Model 2: Göttingen Minipig, pigs are animals whose gastrointestinal tracts are very similar to human ones, and they can be used to optimise and validate the functionality of the device. In both models, it was tested whether the capsule could withstand stomach conditions and arrive at the intestines without trouble. In minipigs, it withstood three weeks inside the gastrointestinal tract. During the tests with minipigs, we saw the need to miniaturise the current device. Currently, we are working on reducing the size
of the pill and all the mechanical components, so the final size goes from 000 to 0.
- Study of the effects of a combination of probiotics on a fatty liver model in rodents
In an initial study the effect of several probiotics in a rat fatty liver model was analysed. To do that, three groups included probiotics Lactobacillus plantarum 748T, Bacillus coagulans GBI-30 6086, and a mixture of the two previous probiotics in their HFHF diet. The three groups showed glycaemia values lower than the untreated group.
In a second study, a microencapsulated and non-microencapsulated Lactobacillus plantarum DSM20174 supplement was used. Mucopenetrating/mucoadhesive capsules were used to achieve the best possible colonisation of the intestine by the probiotic. In this case, the treatment significantly decreased steatosis in regard to the group of rats fed only an HFHF diet, and the levels of glucose in the blood were also lowered. Nevertheless, significant differences were not observed between those who received it with or without microencapsulation.
In both studies, interesting changes were observed in the composition of the intestinal microbiome. - Study of the effects of probiotics on a fatty liver model in minipigs, and validation of the device for taking samples
In the first place, the fatty liver model using an HFFC diet (high fat, high fructose and choline deficient), was induced in Göttingen minipigs. Meanwhile, work was done on microencapsulation of the (L. plantarum) probiotic strain to protect it from the adverse conditions of the stomach, so it can reach the large intestine better. Lastly, the treatment was done according to the following experiment design:
– Grupo 1: 5 Göttingen minipigs fed with an HFFC diet
– Grupo 2: 5 Göttingen minipigs fed with an HFFC + probiotic.
The treatment did not improve the manifestations of the experimental model. Hypercholesterolemia, hypertriglyceridemia and hyperglycemia even worsened. The changes in the intestinal microbiome were not relevant.